Introduction
Material selection stands out as one of the most critical decisions in the development of medical devices.
Choosing the appropriate material ensures the device operates safely, reliably, and functionally. A misstep can undermine its performance, lifespan, and the patient’s safety. At Acrotec Medtech, we recognize that each medical device application poses distinct requirements, and the selected material lays the groundwork for a successful medical device outcome.
This article explores the key factors in selecting materials used in medical devices and highlights the most commonly used materials. It also explains how Acrotec Medtech leverages its broad expertise to help OEMs choose the optimal materials for their specific needs.
How material selection influences medical device safety and performance
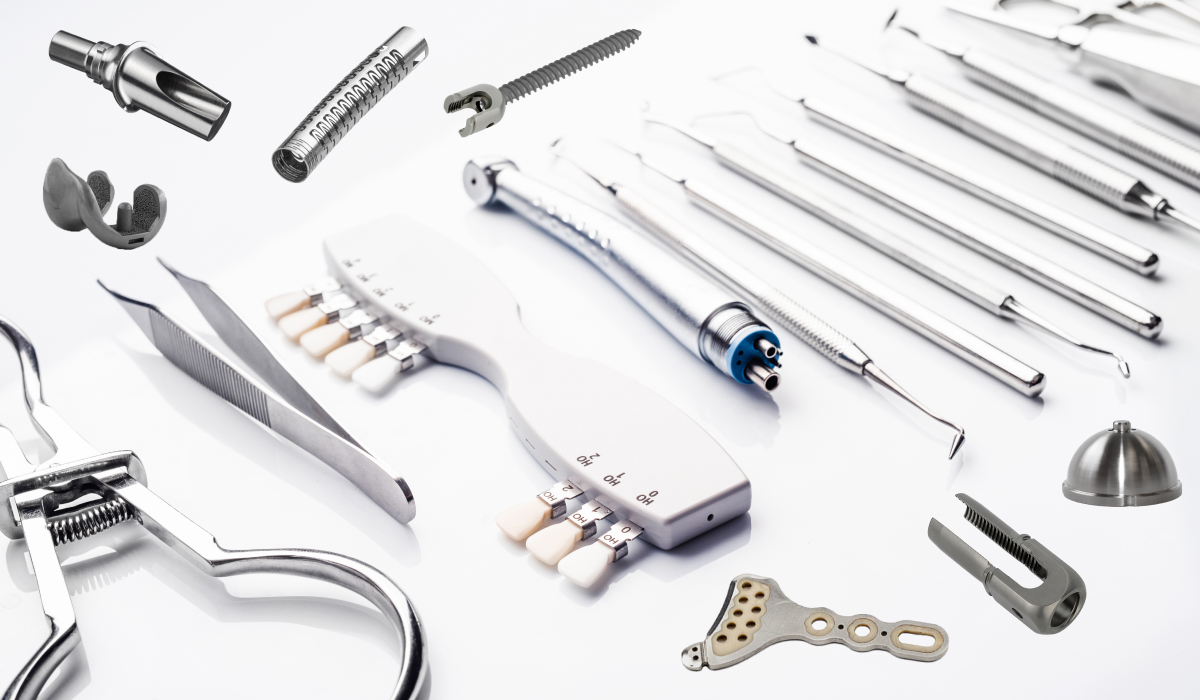
While each material type comes with its own set of unique factors, there are fundamental considerations that concern all materials. These include biocompatibility, sterilization compatibility, regulatory compliance, and corrosion resistance. Such core attributes determine whether a device can function reliably and safely in medical environments.
Functionality and Mechanical Properties
Basic material properties include mechanical strength, elasticity, stiffness, and wear and tear endurance. They are crucial to ensure that the medical device addresses operational demands.
Sterilization Compatibility
Most medical devices require sterilization to prevent infections or residue contamination. Materials should be tested for stability under the intended sterilization methods. These may include autoclaving, ethylene oxide, or gamma irradiation.
Regulatory & Compliance Requirements
Materials must meet regulatory standards set by bodies such as ISO and the FDA. These organizations guide on material safety, performance, and effectiveness. It is therefore essential to stay current with FDA quality system regulations and guidance on biocompatibility, sterilization, and testing. This ensures the use of FDA-approved materials in medical devices.
Manufacturing Process & Cost
Cost efficiency is a priority, as it influences the device’s market position and profitability. Material selection should also align with the chosen manufacturing process (CNC machining, injection molding, additive manufacturing, etc.).
Material Compatibility
When different materials come into contact, they must not corrode or degrade each other’s integrity. This is critical for devices with multi-material assemblies or implants in contact with dissimilar metals.
Biocompatibility
Materials must be compatible with the human body. They need to be:
- Non-immunogenic,
- Non-toxic,
- Non-thrombogenic,
- Non-carcinogenic.
Standards like ISO 10993 and FDA guidance outline tests for cytotoxicity, sensitization, irritation, and systemic toxicity. These tests also define the level of body contact: ranging from limited (external devices) to extended (implants).
Precision
Achieving micrometer‑level tolerances is critical in medical components. The chosen material must allow high‑precision machining to:
- Respond predictably to cutting forces, and
- Retain its shape under stress and temperature changes.
Chemical and Corrosion Resistance
Materials should resist degradation from cleaning agents, disinfectants, and bodily fluids to maintain long-term performance and safety.
Product Shelf Life
Shelf life affects whether a device is designed for single use or multiple sterilization cycles. Material choice should ensure consistent performance over the intended lifespan.
Sustainability & Supply Chain Stability
Emerging considerations include reliable sourcing, lower environmental impact, and recyclability.
These considerations influence the entire medical device lifecycle—from design, prototyping, and testing, to regulatory approval, production, commercialization, and eventual disposal.
Commonly Used Biocompatible Materials in Medical Device Manufacturing

The most commonly used biocompatible materials are metals and polymers. The two categories offer different balances of strength, flexibility, cost, and biocompatibility for various applications.
Metals
Alloys of Stainless Steels
They are the reference medical device materials, mainly for surgical tools and implants.
The most common stainless steels include:
- SAE 316L, a go-to choice for implants, guidewires, and surgical tools,
- SAE 304, widely used for components that demand both strength and formability (e.g. hypodermic needles, endoscopic instruments),
- The martensitic 400-series steels (e.g. SAE 420 and 440), suitable for precision cutting edges and clamping devices,
- 17-4 PH utilized in surgical instruments and specialized chemical handling devices.
Cobalt-chrome alloys
Cobalt–chrome alloys are well recognized in advanced orthopedic devices.
The most common cobalt-chrome alloys include:
- CoCrMo for load-bearing orthopedic implants,
- CoCrWNi for dental prosthetics, stents, and high-wear surgical tools.
Titanium
Commercially pure titanium (CP-Ti) is present in 5 grades:
- Grades 1 and 2, used in surgical instruments and dental implants,
- Grades 3 and 4, ideal for orthopedic implants (hip, joint, shoulders), spinal fusion cages, and trauma fixation plates.
- Grade 5 (Ti-6Al-4V), highly utilized for cardiovascular devices and maxillofacial implants.
Nitinol (Nickel-Titanium Alloy)
Nitinol is used in stents, guidewires, orthodontic archwires, catheters, etc.
Copper
Copper is ideal for high-touch hospital surfaces, pacemakers, defibrillators, imaging equipment, and certain implants such as prostheses and dental implants.
Aluminium
Aluminum is suitable for medical equipment and support devices such as wheelchairs, diagnostic equipment frames, and surgical instruments where lightweight and durability are essential.
It is, however, generally avoided for permanent implants.
Polymers
Medical-Grade Silicone (PDMS)
PDMS is mainly used in long-term implants such as catheters, tubing, and implantable seals.
Polymers
Polymers include:
- Polypropylene (PP), widely used in disposable syringes, specimen containers, and certain surgical trays,
- Polycarbonate (PC) used in housings for surgical instruments, oxygenators, and blood reservoirs,
- Polytetrafluoroethylene (PTFE), employed in vascular grafts, catheter liners, and surgical sutures.
Advanced Polymers
Advanced polymers include:
- The Ultra-High Molecular Weight Polyethylene (UHMWPE), mainly used for joint replacement bearing surfaces, such as acetabular cups and tibial inserts.
- Polyether Ether Ketone (PEEK), widely accepted for implantable applications such as spinal cages, fixation plates, and dental implants.
How Acrotec Medtech Helps OEMs Choose the Right Medical Device Material

Acrotec Medtech offers a single point to a network of specialized companies, each with deep expertise in specific materials and manufacturing techniques.
Its capabilities in micro-machining, surface treatment, and precision manufacturing enable OEMs to develop medical devices from concept through prototyping and full-scale production.
Six key markets are served within the MedTech industry. In each, we apply the medical device component materials best suited for the application. We draw on our network’s expertise to ensure optimal performance, safety, and manufacturability.
Orthopedic Implants (trauma, spine, foot and ankle, dental components)
List of materials
- Titanium and its alloys
- Stainless steel
- PEEK and Carbon-reinforced PEEK
- Cobalt-Chromium alloys
- Low-alloy steel
- Carbon steel
- Tantalum (Ta)
- Advanced ceramics (Zirconia, Alumina-zirconia compound, Zirconia with ESD properties, Silicon nitride)
- Engineering plastics
Ophthalmic and ENT Equipment
List of materials
- Stainless steel
- Aluminum
- Titanium
- Engineering plastics
- Plastics
- Advanced ceramics
Cardiovascular Surgery Implants
List of materials
- Stainless steel
- Titanium and its alloys
- Cobalt-Chromium alloys
- Polymers
- Synthetic jewels (ruby, sapphire, spinel, ceramics)
- Nickel-Titanium alloys (Nitinol)
- Tantalum (Ta)
- Alloy 50 (FeNi50), Fe–Ni–Co alloy (F15)
- Copper-beryllium alloy (Cu-Be)
- Spinels
Surgical Instruments
List of materials
- Stainless steel
- Titanium
- Aluminum
- Polymers
- Silicone
- Carbon steel
- Free cutting steel
- Low-alloy steel
- Engineering plastics
Robotic-Assisted Surgery
List of materials
- PEEK
- Aluminum
- Stainless steel
- Engineering plastics
- Plastics
- Advanced ceramics
Medical Device Components
List of materials
- Stainless Steel
- PEEK Carbon steel
- Aluminum
- Titanium
- Copper
- Tungsten (W)
- Iron (Fe), Electrical steel (FeSi3)
- Alloy 50 (FeNi50), Fe–Ni–Co alloy (F15)
- Copper-beryllium alloy (Cu-Be)
- Spinels
- Plastics & engineering plastics
Conclusion
Materials are the foundation of every medical device, each with its attributes. The key is aligning those properties with the device’s requirements. Regulatory acceptance, sterilization compatibility, and long-term supply stability are considerations that concern all materials.
By understanding not just the individual properties but also how they align with clinical use cases, manufacturers can design devices that meet:
- The functional and safety demands of today’s healthcare environments,
- And the regulatory and durability challenges of tomorrow.
That’s why OEMs need contract manufacturers skilled in working with everything from standard metals to the most advanced alloys and ceramics. Acrotec Medtech brings this expertise through its network of 15 companies, mastering a broad range of materials.
Contact Acrotec Medtech to discover how our material expertise can accelerate your next medical device innovation.
(The summary below groups these companies according to the materials they master.
Metals & Alloys
Titanium
List of companies
- AFT Micromécanique
- Axial Medical
- Dawnlough
- Décovi
- Diener AG Precision Machining (Titanium with anodized finishes)
- EasyDec
- Mebus MIM‑Technik
- Microweld
- Takumi
- Tectri
- Watchdec
Stainless steels
List of companies
- AFT Micromécanique
- Axial Medical
- Dawnlough
- Décovi
- Diener AG Precision Machining
- EasyDec (lead-free)
- Friedrichs Daniels
- Mebus MIM‑Technik
- Microweld
- Takumi
- Team Metal
- Tectri
- Watchdec
Other Steels
List of companies
- Team Metal (Carbon steel, free cutting steel)
- Watchdec (steel)
- Mebus MIM‑Technik (low-alloy steel)
Copper
List of companies
Brass
List of companies
- EasyDec (lead-free brass)
- Microweld
- Team Metal
- Watchdec
Cobalt-chrome alloys
List of companies
Special Materials
List of companies
- Décovi (W)
- Mebus MIM‑Technik (W, F15, Fe, FeSi3, FeNi50)
- Microweld (Nitinol, Ta, W, Cu-Be, Sapphire)
- Pierhor-Gasser (Sapphire, Ruby, Spinels)
- Watchdec (Cu-Be)
Light Metals
Aluminum
List of companies
Polymers & Composites
Engineering Plastics
List of companies
PEEK
List of companies
Plastics
List of companies
Advanced ceramics
List of companies
- Mebus MIM‑Technik (Zirconia, Alumina zirconia compound, Zirconia with ESD properties, Silicon nitride)
- Pierhor-Gasser
Silicone
List of companies



